The Reward and Risk of Expediting COVID-19 Testing and Vaccine Development
U.S. scientists are working quickly to develop new tests and vaccines to identify and combat COVID-19. They have already developed 3 kinds of tests and begun development on numerous potential vaccines.
Developing a new test or vaccine, however, is a complex process. With the reward of quick work also comes risks. Today’s WatchBlog looks at both when it comes to moving rapidly to fight the coronavirus.
COVID-19 testing
In response to the COVID-19 pandemic, the Food and Drug Administration (FDA) has streamlined its regulatory process to get more tests into the hands of medical providers. For example, by using its emergency use authorization authority, FDA has already allowed the use of more than 60 tests.
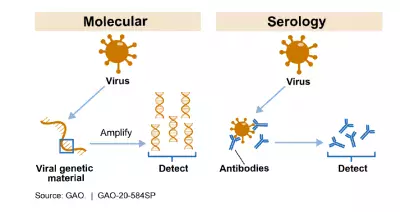
The figure below shows how the 2 most common kinds of tests–molecular and serology–can detect whether someone is currently infected or has been exposed to the virus that causes COVID-19. For example, a molecular test detects the presence of the virus’s genetic material. Serology tests detect the presence of antibodies, which are specific proteins made in response to an infection. Antibodies can be found in the blood of people who have been exposed to the virus.
COVID-19 vaccines
FDA can also use its emergency authority—combined with approaches like Fast Track (a process designed to speed up the review and approval of new therapies for the treatment and prevention of serious or life-threatening conditions such as COVID-19)— to move potential vaccines into clinical trials faster than usual. This could help shorten the amount of time needed to develop a vaccine, helping save lives and accelerate economic recovery.
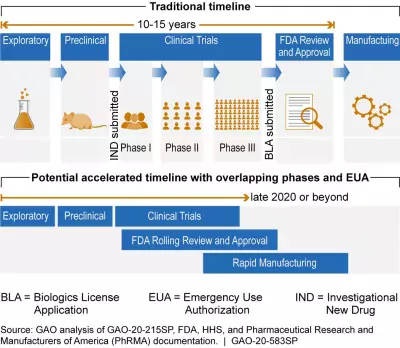
The figure below shows the differences between traditional vaccine development and a potential accelerated timeline.
Note: This graphic was updated on May 28, 2020 to show that phases of vaccine development overlap and clarify accelerated timeline dates.
Risks of moving too quickly
While responding quickly has its benefits, there are also risks.
Accelerating vaccine development could result in less time spent on evaluating the safety of the vaccine. Some patients could experience serious adverse effects. Shortening the timeline could also mean that scientists learn less about the potential range of adverse effects.
Moving quickly to authorize and distribute tests may increase risks associated with their accuracy. As a result, there is the potential that some tests could show patients have not been exposed to or infected by the virus that causes COVID-19 when they actually have been. In addition, while tests may be developed quickly, their widespread use could be limited by the availability of supplies required to conduct testing safely, including swabs and personal protective equipment.
Finding the right balance between speed and safety, accuracy, and effectiveness is challenging. It requires careful assessment and may need to be adjusted over time.
Want to know more? Check out our Science & Tech Spotlights on COVID-19 Testing and COVID-19 Vaccine Development. GAO has a broad portfolio of work on the federal government’s response to and preparedness for infectious diseases, viruses, and biological threats including Zika, Ebola, Avian Influenza, and H1N1.
Comments on GAO’s WatchBlog? Contact blog@gao.gov.
GAO Contacts
Related Products

GAO's mission is to provide Congress with fact-based, nonpartisan information that can help improve federal government performance and ensure accountability for the benefit of the American people. GAO launched its WatchBlog in January, 2014, as part of its continuing effort to reach its audiences—Congress and the American people—where they are currently looking for information.
The blog format allows GAO to provide a little more context about its work than it can offer on its other social media platforms. Posts will tie GAO work to current events and the news; show how GAO’s work is affecting agencies or legislation; highlight reports, testimonies, and issue areas where GAO does work; and provide information about GAO itself, among other things.
Please send any feedback on GAO's WatchBlog to blog@gao.gov.