Animal Use in Research: Federal Agencies Should Assess and Report on Their Efforts to Develop and Promote Alternatives
Fast Facts
Researchers often use animals to study disease, test product safety, experiment, or teach. Some uses cause animals pain or distress. Federal agencies require researchers to consider alternatives to animal use, such as computer modeling or working with cell cultures.
The Department of Health and Human Services and other agencies have developed animal use alternatives and collaborate on these efforts in an interagency group. However, the agencies don’t routinely measure the effect of those efforts. We recommended that HHS create a workgroup to help agencies assess and report on their progress in reducing or replacing animal use in research.
This “tissue chip,” which is lined by living cells, can be used as an alternative to animal testing.
Small wires and materials over light source
Highlights
What GAO Found
The Department of Health and Human Services (HHS), U.S. Department of Agriculture (USDA), and Environmental Protection Agency (EPA) use a variety of methods to ensure researchers consider alternatives to animal use in research (see figure). Two of these methods are (1) requiring researchers to obtain approval of their research protocols, including their consideration of alternatives, from their institutions, and (2) calling for or recommending researchers to use database searches to identify alternatives. HHS and USDA also help ensure that researchers consider alternatives through the agencies' oversight of research facilities. For example, USDA is to conduct annual inspections of nonfederal research facilities. Futhermore, the agencies have provided training to researchers on the consideration of alternatives.
HHS, USDA, and EPA have facilitated the development and use of alternatives to animal use in research through individual and collaborative efforts. These efforts include agency strategies and policies for promoting the use of alternative methods and the development of testing methods that rely on non-animal models. Additionally, the agencies are members of the Interagency Coordinating Committee on the Validation of Alternative Methods, which is managed by HHS's National Institute of Environmental Health Sciences. The committee promotes testing methods that protect human health and the environment while reducing animal use. The interagency committee's 2018 strategic roadmap calls for it to identify appropriate metrics for monitoring progress and measuring success in adopting alternatives. However, the committee and its member agencies have not routinely developed or reported metrics that demonstrate how their efforts to encourage the use of alternative methods affect animal use. They have also not designated an interagency workgroup to address the challenges related to developing and reporting such metrics. Facilitating the establishment of such a workgroup would help the committee and its member agencies better monitor their progress across the range of their efforts to reduce animal use and report members' progress to the public.
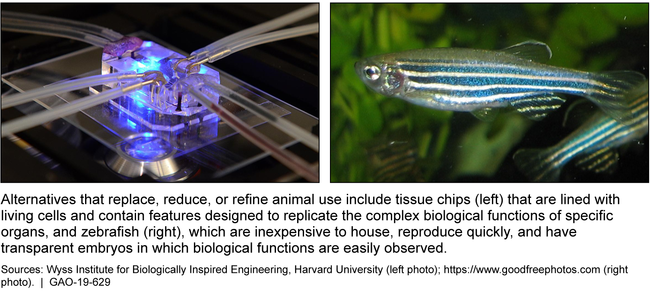
Examples of Methods to Replace, Reduce, or Refine Animal Use in Research
Why GAO Did This Study
U.S. research facilities use a wide range of animal species in research, and some procedures used during such research cause animals pain or distress. Federal laws and principles require that researchers consider alternatives to animal use, such as in vitro methods and computer modeling. HHS, USDA, and EPA conduct and fund animal research and regulate products tested on animals. HHS and USDA also oversee federal and nonfederal research facilities including researchers' consideration of alternatives to animal use.
GAO was asked to review issues related to alternatives to animal research. This report (1) describes how HHS, USDA, and EPA ensure researchers consider the use of alternatives to animals and (2) examines the steps the agencies have taken to facilitate the use of alternative research methods and to assess the effect of their efforts on animal use. GAO reviewed documents, such as agency policies and practices relevant to the consideration of alternatives and interviewed agency officials. GAO also interviewed representatives of a nongeneralizable sample of 12 federal and nonfederal research facilities randomly selected across agencies and facilities.
Recommendations
GAO recommends that HHS's National Institute of Environmental Health Sciences facilitate the establishment of an interagency workgroup to develop metrics for assessing progress on the development and promotion of alternatives to animal use and incorporate those metrics into public reports. HHS agreed with our recommendation.
Recommendations for Executive Action
| Agency Affected | Recommendation | Status |
|---|---|---|
| National Institute of Environmental Health Sciences | The Director of the NIH's National Institute of Environmental Health Sciences should (1) facilitate the establishment or designation of a workgroup of representatives of the Interagency Coordinating Committee on the Validation of Alternative Methods member agencies to develop metrics that the agencies could use to assess the progress they have individually or collectively made toward reducing, refining, or replacing animal use in testing and (2) incorporate those metrics into the committee's biennial progress reports. (Recommendation 1) |
The Director of the NIH's National Institute of Environmental Health Sciences asked the lead NIH official for the Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM) to lead the efforts necessary to implement this recommendation. In turn, in November 2019, ICCVAM member agencies formed a workgroup comprised of individuals within their respective agencies who are best suited to developing agency-specific metrics. The workgroup held its first meeting in February 2020 with an aim to develop metrics, for individual agency application, of progress toward reducing, refining, or replacing animal use in testing. In its biennial progress report for 2018 and 2019, issued in July 2020, ICCVAM included metrics for two member agencies, the U.S. Department of Agriculture and Environmental Protection Agency, including metrics for reductions in the number of animal tests through the use of alternatives.
|
