Regenerative Medicine: Therapeutic Applications, Challenges, and Policy Options
Fast Facts
Regenerative medicine restores or replaces human cells, tissues, or organs affected by disease. This may eventually help cure many conditions that are currently considered difficult to treat.
For example, a patient's own cells could be used to create a personalized organ, which could eliminate the possibility that a patient's body will reject a donated organ.
However, there are challenges with developing regenerative medicine technologies. We offer 11 policy options to help address such challenges. For example, federal agencies could help by supporting public-private partnerships that can share costs for manufacturing facilities.

Highlights
What GAO Found
Regenerative medicine offers the hope of being able to restore or replace cell, tissue, and organ functions affected by disease, injury, or aging. This may eventually help manage or cure many conditions that are currently considered chronic, untreatable, or terminal.
Examples of Diseases and Regenerative Medicine Therapies That Might Address Them
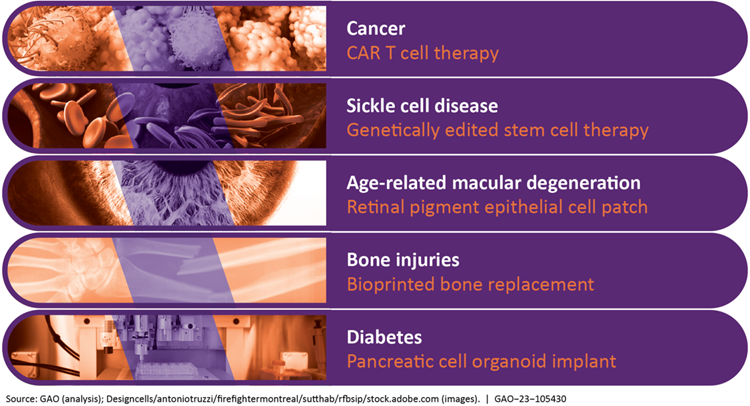
GAO identified many challenges that may affect the development and use of regenerative medicine technologies and therapies including:
Challenges related to standardization. Standards are rules, conditions, guidelines, or agreed-upon practices that are adopted within an industry to provide developers with a common framework and promote consistency. Developing regenerative medicine standards is challenging because these technologies and therapies are complex and rapidly evolving. In addition, standards require consensus from stakeholders, which may be difficult to obtain.
Challenges related to regulation. The Food and Drug Administration (FDA) ensures the safety, efficacy, and security of human medical products in the U.S. through regulation. Regenerative medicine faces challenges related to regulation, including difficulty navigating a complex regulatory framework, uncertainty over which regulatory pathway is most appropriate for certain emerging technologies and therapies, and staffing shortages at FDA and collaborating agencies.
Challenges related to manufacturing. Manufacturing is the creation of products from starting materials, in a way that is generally consistent and reproducible. It is a key step for many emerging technologies and therapies, but the cells, tissues, and organs used for regenerative medicine are complex and difficult to manufacture at scale. Other challenges to manufacturing include a lack of infrastructure and difficulty ensuring quality and consistency.
GAO developed 11 policy options that could help address the challenges or enhance the benefits of regenerative medicine. These policy options are provided to inform policymakers of potential actions to address the policy challenges identified in this technology assessment. They identify possible actions by policymakers, which include Congress, federal agencies, state and local governments, academic and research institutions, and industry. Policymakers would need to consider the impacts these new technologies will have on existing federal programs that are already strained. We suggested possible federal components for the policy options.
Selected Policy Options to Mitigate Challenges Associated with Regenerative Medicine Technologies and Therapies
| Selected policy Option | Opportunities | Considerations |
|---|---|---|
|
Invest in standards development.
|
|
|
|
Provide opportunities for increased interactions between regulatory experts (at FDA or in industry) and smaller companies, especially early in the development process (report p. 32)
|
|
|
|
Consider whether changes to the framework for evaluating combination products and medical devices to accommodate emerging technologies and therapies may be necessary. (report p. 33)
|
|
|
|
Provide more oversight and feedback to suppliers to increase consistency in starting materials (report p. 40)
|
|
|
Source: GAO. | GAO-22-105274
Why GAO Did This Study
Regenerative medicine represents a paradigm shift in the medical field because it aims to restore or supplement function, rather than just treating symptoms, and opens the door for personalized therapies. GAO conducted an assessment of current and emerging regenerative medicine technologies and therapeutic applications. This report examines (1) current and emerging regenerative medicine technologies and therapies and their potential benefits, (2) challenges that hinder their development and use, and (3) policy options that could help enhance benefits and mitigate challenges associated with these technologies and therapies. GAO reviewed scientific and policy literature and other key reports; convened a 3-day expert meeting; interviewed subject matter experts and stakeholder groups including government agencies, such as the Department of Health and Human Services, non-government organizations, industry, academia, end user groups such as patient groups; and conducted site visits. GAO is identifying policy options in this report.
For more information, contact Karen L. Howard at (202) 512-6888 or howardk@gao.gov.
