Brain-Computer Interfaces: Applications, Challenges, and Policy Options
Fast Facts
This technology assessment examines systems that are implanted in the brain or worn on the head that let people control electronic devices using brain signals.
In clinical trials, these brain-computer interfaces helped people with severe disabilities. Businesses are also investing in developing the technology for entertainment and other uses.
We identified challenges with this fast-moving technology. These include uncertainty over:
Who owns sensitive brain data
How to support people with implanted devices over the long term
What Medicare and private insurers will cover
We outlined policy options to help address these issues and more.
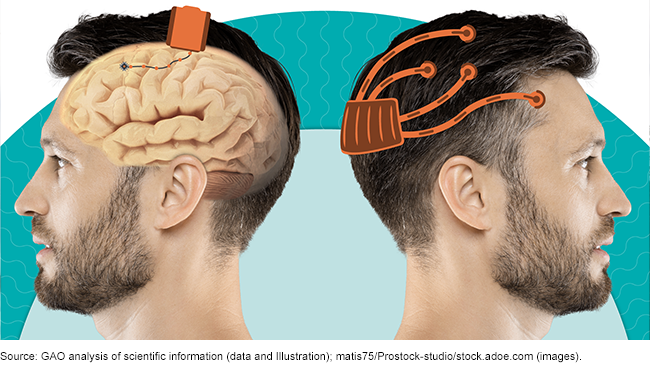
Implantable and wearable brain-computer interfaces
Highlights
What GAO Found
Brain-computer interfaces (BCI) are electronic systems—either implanted in the brain or worn on the head—that let people control computers, robots, or other devices using brain signals. In clinical trials, BCIs have helped people with severe disabilities communicate and use robotic limbs, though these BCIs are not yet on the market. Researchers are also investigating—and companies are investing heavily in— BCIs for the workplace, national defense, and consumer uses.

Experts identified several challenges to BCI development and use, including:
Uncertainties in data ownership and control. Without a unified privacy framework for all BCIs, or standards on data ownership and control, companies that develop and sell BCIs may have access to sensitive brain signal data without users' understanding or consent. In addition, agreements between developers and users may be predatory or unclear.
Potential loss of access or support. Experts told us that users may lose access to the benefits of their implanted BCIs for various reasons. For example, some clinical trial participants have had a BCI removed because there were no funds or medical support provided after the trial. Experts said there is a need to prioritize support and maintenance for participants after a trial or if a developer ceases operation.
Medicare coverage decision process. The Centers for Medicare & Medicaid Services (CMS) makes coverage determinations for Medicare. Private insurers and other public programs may use CMS decisions as a guide for their own coverage. Experts told us that it can be challenging to interact with CMS about BCIs. Officials said CMS has provided a specific point of contact to facilitate early dialogue between developers and reviewers and has improved guidance for navigating CMS processes for determining coding, coverage, and payment.
GAO developed eight policy options that could help address the challenges described above. The options identify possible actions by policymakers, including legislative bodies, government entities, academia, industry, and other groups. In addition, policymakers could choose to maintain the status quo, whereby they would not take additional action beyond current efforts. Some of the policy options are included below. See tables 3–6 in this report for additional policy options and details.
Selected Policy Options to Mitigate Challenges Associated with Brain-Computer Interfaces (BCIs)
|
Selected policy option |
Opportunities |
Considerations |
|---|---|---|
|
Provide consumers with more control over the use of their data, including brain signal data and other data associated with use of a BCI (report p. 18). This policy option could help address uncertainties in data ownership and control. |
|
|
|
Consider options for protecting brain signal and other data associated with use of a BCI (report p. 19). This policy option could help address uncertainties in data ownership and control. |
|
|
|
Prioritize device maintenance and support for users (report p. 23). This policy option could help address the challenge faced by users who may lose access to, or support for their BCI. |
|
|
|
Consider strategies to increase coordination between BCI developers and the Centers for Medicare & Medicaid Services (CMS). (report p. 24). This policy option could help address the challenge of CMS coverage decision processes being a potential key hindrance to adoption. |
|
|
Source: GAO. | GAO-25-106952
Why GAO Did This Study
BCIs may offer quality-of-life improvements for people living with disabilities due to neurological disorders, stroke, or injuries. BCIs also have emerging nonmedical uses in the workplace, national defense, and entertainment.
With rapid progress in BCI development, policymakers may want to consider how best to support this technology while also ensuring quality medical care and protecting users—both of medical and nonmedical BCIs.
This technology assessment examines (1) BCI technologies available or in development, along with their potential benefits, (2) challenges to the development and use of BCIs, and (3) options policymakers could consider to help address the challenges.
To conduct this work, GAO reviewed scientific literature and federal agency guidance. GAO also interviewed federal agency officials and other experts from government, academia, industry, nonprofit organizations, and end user groups. GAO is identifying policy options in this report.
For more information, contact Karen L. Howard at (202) 512-6888 or HowardK@gao.gov.
