COVID-19: Information on HHS's Medical Countermeasures Injury Compensation Program
Fast Facts
Most people who receive vaccines or treatments for certain public health threats, such as COVID-19 vaccines, have no serious problems as a result. But with any medicine, there is a rare chance of injury or death.
To encourage the development of these vaccines and treatments, legislation limited industry liability. It also authorized a compensation program for serious injuries or deaths.
The program:
Received a surge of 13,333 COVID-19 claims—27 times the number of claims received in the first decade of the program
Completed a review of about a fourth of all claims
Found that 92 (3%) of the completed claims were eligible for compensation
Highlights
What GAO Found
Medical countermeasures, such as vaccines and drugs used to treat COVID-19, can save lives when there is a public health emergency or threat. Most people who receive a countermeasure have no serious problems, but like any medicine, there is a rare chance that some can cause serious injuries or deaths, according to the Department of Health and Human Services (HHS).
To encourage the development of medical countermeasures, the Public Readiness and Emergency Preparedness Act limited the legal liability of manufacturers and others for losses related to the administration or use of covered countermeasures. It also authorized HHS to establish the Countermeasures Injury Compensation Program (CICP) to compensate individuals who die or suffer serious physical injuries directly caused by the administration or use of certain medical countermeasures.
CICP is operated by the Health Resources and Services Administration (HRSA)—an agency within HHS. To be eligible for compensation, individuals must file a claim within one year of the administration or use of a covered countermeasure and provide medical documentation to support that the countermeasure directly caused a serious physical injury or death.
HRSA data show that since CICP began accepting claims in October 2009, the program received about 27 times more claims in response to the COVID-19 pandemic than in the first decade of the program—13,333 compared to 491 claims, respectively.
Countermeasures Injury Compensation Program Total Claim Submissions , by Fiscal Year, as of June 2024
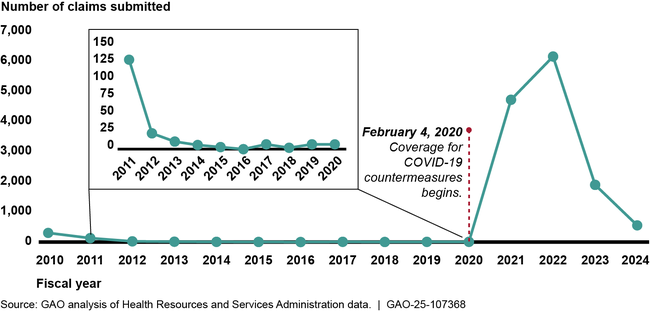
HRSA made decisions on 25 percent of the total claims submitted as of June 2024 (the most recent data available at the time of GAO’s analysis); the remaining 75 percent of claims were under review or pending review. Of the claims that have completed HRSA’s adjudication process, about 3 percent (92) were found eligible to receive compensation. Fifty-six percent of the paid claims were COVID-19-related. HRSA data show that it took the agency 24 months on average to complete both the initial eligibility check and medical review needed to make a claim decision. For claims found ineligible after the initial eligibility check, HRSA took 14 months on average, as of June 2024. Missing the filing deadline was the most common reason for ineligible claim decisions. HRSA paid roughly $6.5 million in compensation for eligible claims as of June 2024, with most of that amount for serious injuries, such as Guillain-Barré syndrome, caused by the H1N1 vaccine. About $400,000 was paid for injuries related to COVID-19 countermeasures, such as myocarditis (inflammatory heart condition).
Nearly all of the challenges HRSA experienced operating CICP stem from the large influx of claims related to COVID-19 medical countermeasures and limited resources to process and pay claims prior to fiscal year 2022, HRSA officials told GAO. Specifically:
- shortage of staff to adjudicate the large influx of claims;
- outdated information systems to process the large number of claims; and
- limited medical and scientific evidence to base decisions about injuries or deaths allegedly caused by novel COVID-19 countermeasures.
To address these challenges, HRSA hired more staff and launched a web portal for online claims submissions, among other things. HRSA also started developing a COVID-19 Countermeasure Injury Table—a table that lists injuries that are presumed to be caused by COVID-19 countermeasures—to help streamline claims reviews. HRSA planned to publish the table in a proposed rule by November 2024, according to the most recent Unified Agenda of Regulatory and Deregulatory Actions.
Thirty-eight foreign countries operate medical injury compensation programs, nine of which began during COVID-19, according to research. There are also three international programs. According to program documents, these programs primarily focus on harm from vaccines. Officials from three of the four foreign countries’ medical injury compensation programs in GAO’s review said they have taken or plan to take actions in response to their experiences operating during the COVID-19 pandemic, such as creating contracts that allow programs to quickly hire staff to scale up capacity when needed.
Why GAO Did This Study
The CARES Act includes a provision for GAO to report on the federal government’s ongoing monitoring and oversight efforts related to the COVID-19 pandemic. This report describes CICP, including its claims adjudication process, and similar programs in selected foreign countries.
GAO analyzed HRSA data on CICP claims submitted from October 2009 (the date the program began accepting claims) through June 2024 (the most recent data available). GAO reviewed relevant HRSA documentation and interviewed agency officials. GAO further reviewed documents and interviewed officials from a non-generalizable sample of five foreign medical injury compensation programs—four from foreign countries (Canada, Singapore, Sweden, and Switzerland) and one international program (COVAX No-Fault Compensation Program for Advanced Market Commitment Eligible Economies). GAO selected these countries for variation in the length of program operation and the claim filing deadlines, and variation in the country’s COVID-19 vaccine administration rates. GAO selected the international program because it covered the largest number of countries.
For more information, contact Mary Denigan-Macauley at (202) 512-7114 or DeniganMacauleyM@gao.gov.
